Working as a team, scientists from SIMES and the SUNCAT Center for Interface Science and Catalysis studied two platinum-rhodium nanostructures, identifying the most active sites on the platinum islands and predicting that an optimized platinum-rhodium nanostructure could be up to five times more active than pure platinum.
Researchers from two SLAC-Stanford joint institutes, the Stanford Institute for Materials and Energy Sciences (SIMES) and the SUNCAT Center for Interface Science and Catalysis, recently joined forces to investigate a catalyst that promotes energy-releasing reactions in fuel cells.
They discovered that the catalyst, made of ultrathin platinum layers grown on a single crystal of rhodium, would work better and last longer if its structure – as well as its composition – was carefully engineered.
Stable, highly active catalysts are particularly needed in fuel cells, which could power electric vehicles without the range limitations of current batteries. But the catalysts are often made of scarce and expensive materials like platinum. Attempts to engineer such catalysts have failed, however, because the most active catalysts are not stable enough, and the most stable catalysts aren’t active enough.
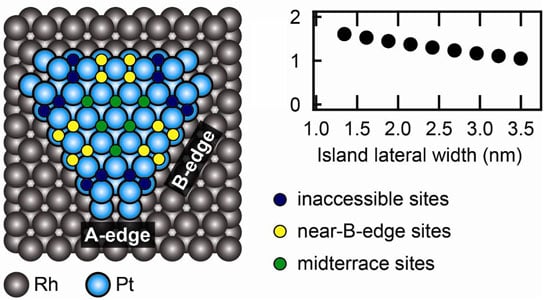
The SIMES team, led by Associate Staff Scientist Daniel Friebel, studied two very different platinum-rhodium nanostructures: one with platinum deposited on the rhodium crystal in a single, atom-thick layer, and the other with the same amount of platinum forming thicker islands with bare rhodium in between. They used the high-resolution X-ray spectrometer at the Stanford Synchrotron Radiation Lightsource’s Beam Line 6-2 to examine the surface chemistry that determines the catalytic activity of both structures.
The two samples showed markedly different behavior: The platinum islands were much better at grabbing and holding oxygen atoms than the platinum monolayer, which captured almost no oxygen on its surface. In fuel cells, atomic oxygen is the key intermediary between bond-breaking and bond-making chemical reactions on a fuel cell’s cathode, where oxygen molecules, current-carrying electrons, and protons that have been generated from hydrogen at the fuel cell’s anode are transformed into water.
How strongly oxygen atoms are held by the catalyst is a vital consideration when determining its efficacy, said Friebel. “If the oxygen is too weakly attached to the catalyst, the initial bond-breaking never gets going. If, however, oxygen gets stuck, it will throttle the bond-making that is needed to complete the reaction.”
Venkat Viswanathan, a graduate student at SUNCAT, was able to explain the SIMES results using a theoretical tool called density functional theory. For each platinum surface atom he found a simple description of its ability to grab oxygen, which depends on how many platinum and rhodium atoms are in its immediate neighborhood. Generally, platinum atoms with fewer neighboring metal atoms can bind oxygen more strongly, but where a neighbor atom exists, another platinum atom is preferred. A rhodium neighbor spoils platinum’s appetite for oxygen more than a platinum neighbor.
This interaction between platinum and rhodium is why the thicker platinum islands bind oxygen more strongly than the atom-thick platinum layer, but still more weakly than pure platinum.
Researchers were also able to identify the most active sites on the platinum islands and predict that an optimized platinum-rhodium nanostructure could be up to five times more active than pure platinum. Moreover, such a structure is expected to resist degradation much better than platinum-nickel or platinum-cobalt catalysts with comparable activity, thus fulfilling requirements for both high activity and stability.
Although rhodium, like platinum, is too expensive to use as a catalyst, the knowledge gained from these studies enables novel approaches to inexpensive catalyst design.
The SIMES-SUNCAT research appeared in the Journal of the American Chemical Society.
Reference: “Balance of Nanostructure and Bimetallic Interactions in Pt Model Fuel Cell Catalysts: In Situ XAS and DFT Study” by Daniel Friebel, Venkatasubramanian Viswanathan, Daniel J. Miller, Toyli Anniyev, Hirohito Ogasawara, Ask H. Larsen, Christopher P. O’Grady, Jens K. Nørskov and Anders Nilsson, 22 May 2012, Journal of the American Chemical Society.
DOI: 10.1021/ja3003765