
Scientists reveal three unique electron states in molten salts, a crucial discovery for future salt-fueled nuclear reactors’ radiation impacts.
In a finding that helps elucidate how molten salts in advanced nuclear reactors might behave, scientists have shown how electrons interacting with the ions of the molten salt can form three states with different properties. Understanding these states can help predict the impact of radiation on the performance of salt-fueled reactors.
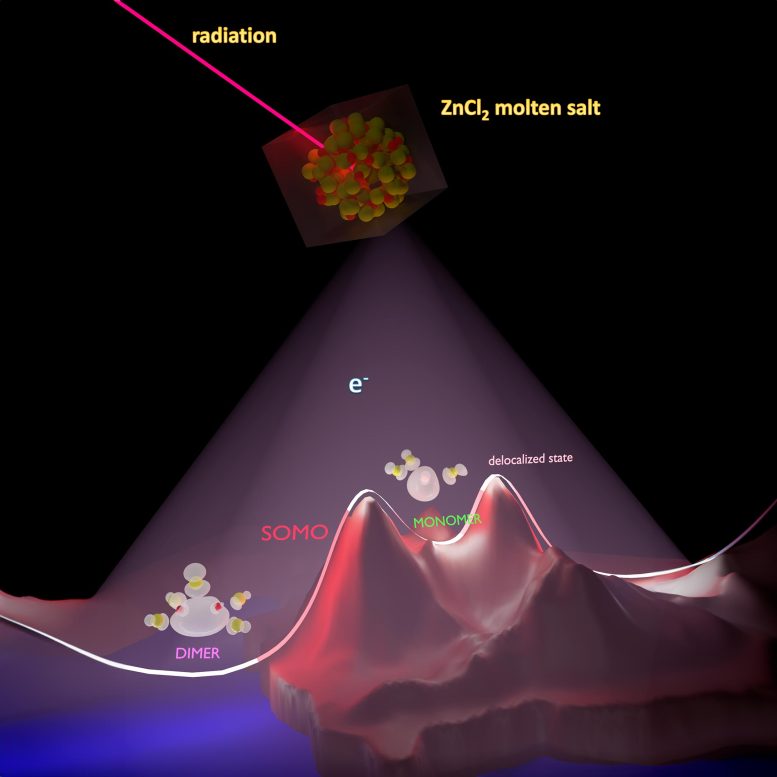
The researchers, from the Department of Energy’s Oak Ridge National Laboratory and the University of Iowa, computationally simulated the introduction of an excess electron into molten zinc chloride salt to see what would happen.
They found three possible scenarios. In one, the electron becomes part of a molecular radical that includes two zinc ions. In another, the electron localizes on a single zinc ion. In the third, the electron is delocalized, or spread out diffusely over multiple salt ions.
Implications for Future Reactor Designs
Because molten salt reactors are one of the reactor designs under consideration for future nuclear power plants, “the big question is what happens to molten salts when they’re exposed to high radiation,” said Vyacheslav Bryantsev, leader of the Chemical Separations group at ORNL and one of the scientists on the study and an author of the paper. “What happens to the salt that is used to carry the fuel in one of those advanced reactor concepts?”
Claudio Margulis, professor of chemistry at the University of Iowa and also a study investigator and author, said, “Figuring out how the electron interacts with salt is important. We see from the study that, at very short times, the electron can facilitate the formation of a zinc dimer, a monomer, or be delocalized. It is conceivable that on longer time scales such species could further interact to form other more complex ones.”
In this study, the scientists wanted to understand how an electron, which appears because of radiation generated by nuclear fuel or other energy sources, will react with the ions that make up a molten salt.
“This study doesn’t answer all these questions, but it’s a start to investigate more deeply how the electron interacts with the salt,” Margulis said.
Potential Long-Term Interactions and Published Findings
Margulis continued: “Since our first-principles molecular dynamics calculations show that these three species can form in the melt at very short times, it begs the question of what other species can form at longer times. We do not have an answer for this. One option is that the electrons can return to the species where they came from; for example, a chlorine radical can take back an electron to form chloride. Another is that radical species may react in more complex ways. Of particular interest is the case when radiation generates enough radicals that these can be in close proximity; this is when they could react to form more complex species.”
The researchers, along with Iowa graduate student Hung Nguyen, published their findings in the American Chemical Society’s The Journal of Physical Chemistry B. The paper, “Are High-Temperature Molten Salts Reactive with Excess Electrons? Case of ZnCl2,” was chosen as an ACS Editors’ Choice, an honor bestowed on one paper from across the entire ACS portfolio that has particular potential for broad public interest. It has also been selected for the journal’s front cover.
The research was part of DOE’s Molten Salts in Extreme Environments Energy Frontier Research Center, or MSEE EFRC, led by Brookhaven National Laboratory. An EFRC is a basic research program funded by DOE’s Office of Basic Energy Sciences that brings together creative, multidisciplinary, and multi-institutional teams of researchers to address the toughest grand scientific challenges at the forefront of fundamental energy science research.
The Broader Significance
“This research is important because it shows how the excess electrons generated by radiation in molten salt reactors could have multiple forms of reactivity. I and other members of the MSEE team are attempting to identify these other forms of reactivity experimentally,” said Brookhaven chemist James Wishart, director of the MSEE EFRC.
“This study can give us some understanding of how an electron can interact with a molten salt,” Bryantsev said. “There are a lot of questions still open. For example, is this interaction similar to what happens with other salts?”
Nguyen, the paper’s first author, said, “I continue to work with Professor Margulis, Dr. Bryantsev, as well as other members of the MSEE project to extend our studies by looking at other salt systems. Hopefully, we will be able to answer more questions on the effect of radiation on molten salts.”
Reference: “Are High-Temperature Molten Salts Reactive with Excess Electrons? Case of ZnCl2” by Hung H. Nguyen, Vyacheslav S. Bryantsev and Claudio J. Margulis, 27 September 2023, The Journal of Physical Chemistry B.
DOI: 10.1021/acs.jpcb.3c04210
The computational research was done at DOE’s Compute and Data Environment for Science at ORNL and the National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory, both DOE Office of Science user facilities.
1 Comment
When did the salt become the fuel ? (“salt fueled” reactors). We have enough people that are unqualified writing about technical issues, making mistakes.
Why “researching” zinc chloride salt ? Who need it & who plans on using it ?
We need to get the MSR’s manufactured & installed, using the salt & fuel we already have and have tested. If China can do it, the U.S. can do it !!!
The “researchers” and bureaucrats at the Nuclear Reg. Commission need to learn how to grow food and work on farms.