Researchers discovered a novel antibacterial mechanism of plectasin, an antibiotic derived from a fungus. The study reveals that plectasin forms Velcro-like structures that trap crucial bacterial components, preventing their escape and enhancing drug effectiveness. This mechanism could guide the development of new antibiotics to fight antimicrobial resistance.
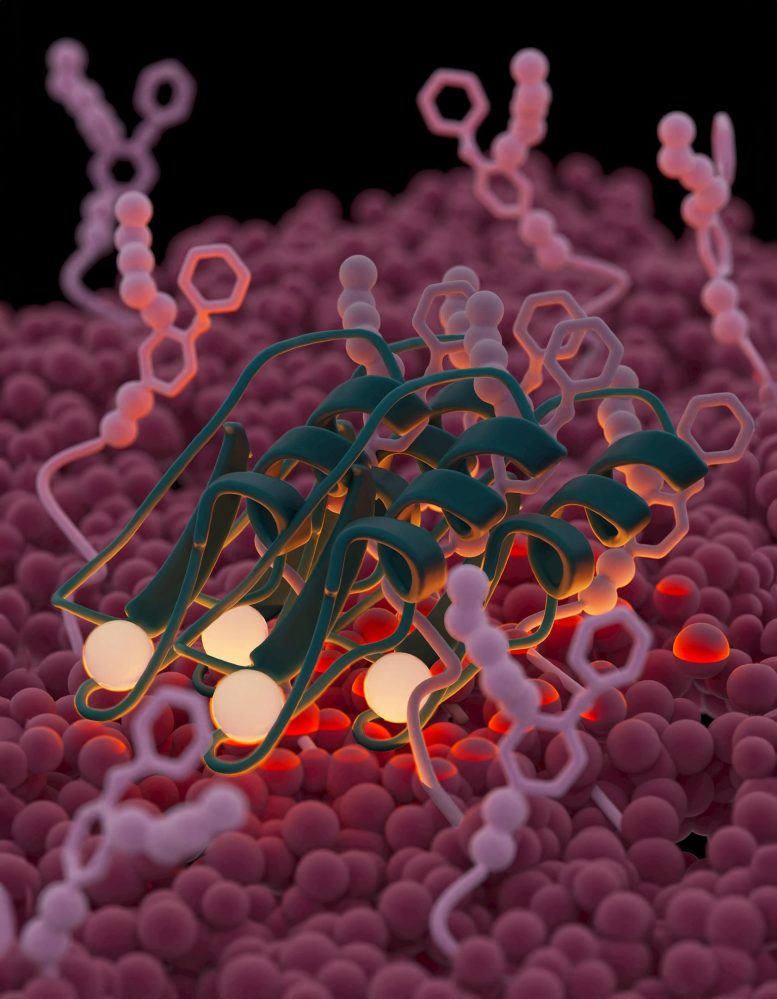
Plectasin, a small antibiotic, uses an innovative mechanism to kill bacteria. It assembles to form a large structure that latches onto its target on the bacterial cell surface comparable to how both sides of Velcro form a bond.
A research team mapped how the Velcro-structure is formed. Their discovery unveils a new approach that could have broad implications for the development of antibiotics to combat antimicrobial resistance. Published today (May 23) in the scientific journal Nature Microbiology, the research was led by structural biologist Markus Weingarth and biochemist Eefjan Breukink at Utrecht University.
Innovative Research Techniques
The research team delved into the workings of plectasin, an antibiotic derived from the fungus Pseudoplectania nigrella. The scientists employed advanced biophysical techniques, including solid-state NMR and, in collaboration with Wouter Roos from Groningen, atomic force microscopy.
Traditionally, antibiotics function by targeting specific molecules within bacterial cells. However, the mechanism behind plectasin’s action was not fully understood until now. Previous studies suggested a conventional model where plectasin binds to a molecule called Lipid II, crucial for bacterial cell wall synthesis, akin to a key fitting into a lock.

Velcro-Like Structures in Antibacterial Action
The new study reveals a more intricate process. Plectasin doesn’t just act like a key in a lock; instead, it forms dense structures on bacterial membranes containing Lipid II. These supramolecular complexes trap their target Lipid II, preventing it from escaping. Even if one Lipid II breaks free from plectasin, it remains contained within the Velcro-structure, unable to escape.
Weingarth compares this structure to Velcro, where plectasin forms the microscopic hooks that attach to bacterial ‘loops’. In normal Velcro, if one of the loops breaks free from its hook, it is still trapped by the entire structure. The same goes for bacteria trapped in the plectasin superstructure: they can break free from the plectasin’s binding, but stay trapped in the superstructure. This prevents the bacteria to escape and cause further infections.
Role of Calcium Ions in Plectasin’s Effectiveness
Moreover, the researchers found that the presence of calcium ions further enhances plectasin’s antibacterial activity. These ions coordinate with specific regions of plectasin, causing structural changes that significantly improve the antibacterial effectiveness. That ions play a critical part in the action of plectasin was discovered by PhD students Shehrazade Miranda Jekhmane and Maik Derks, co-first authors of the study. They realized that plectasin samples had a peculiar color, which hinted at the presence of ions.
Implications for Future Antibiotic Development
Markus Weingarth, the lead author of the study, expects this finding could open new avenues for developing superior antibiotics.
“Plectasin is presumably not the ideal antibiotic candidate due to safety concerns. However, in our study, we show that the ‘Velcro-mechanism’ appears widely used among antibiotics, which was thus far ignored. Future drug design efforts hence not only need to focus on how to bind targets, but also how drugs can self-assemble efficiently. Thereby, our study closes a major knowledge gap which could have broad implications for the design of better drugs to combat the growing threat of antimicrobial resistance.”
Reference: “Host defence peptide plectasin targets bacterial cell wall precursor lipid II by a calcium-sensitive supramolecular mechanism” by Shehrazade Jekhmane, Maik G. N. Derks, Sourav Maity, Cornelis J. Slingerland, Kamaleddin H. M. E. Tehrani, João Medeiros-Silva, Vicky Charitou, Danique Ammerlaan, Céline Fetz, Naomi A. Consoli, Rachel V. K. Cochrane, Eilidh J. Matheson, Mick van der Weijde, Barend O. W. Elenbaas, Francesca Lavore, Ruud Cox, Joseph H. Lorent, Marc Baldus, Markus Künzler, Moreno Lelli, Stephen A. Cochrane, Nathaniel I. Martin, Wouter H. Roos, Eefjan Breukink and Markus Weingarth, 23 May 2024, Nature Microbiology.
DOI: 10.1038/s41564-024-01696-9