A team from Osaka University, along with their collaborators, has created a cost-effective catalyst for a key chemical process, potentially paving the way for further initiatives to reduce expenses within the chemical sector.
The chemical industry often relies on scarce and costly metals to manufacture pharmaceuticals and other crucial materials. Substituting these metals with more readily available and affordable alternatives could enhance environmental sustainability, reduce expenses, and decrease the likelihood of supply chain interruptions.
Now, in a study recently published in Chemistry – A European Journal, researchers from Osaka University and collaborating partners have met this need in their work on an industrially useful chemical transformation. The simple, gentle reaction conditions reported here might inspire researchers who are working to reduce use of expensive metals for as many chemical reactions as possible.
The Role of Noble Metals and Alternatives
So-called noble metals are especially versatile materials. For example, palladium is a metal of choice for catalyzing a chemical transformation – converting nitriles into primary amines – that is a common step in nylon and plastics production. However, such metals are rare and costly. Substitutes based on common metals such as nickel could be cheaper catalysts.
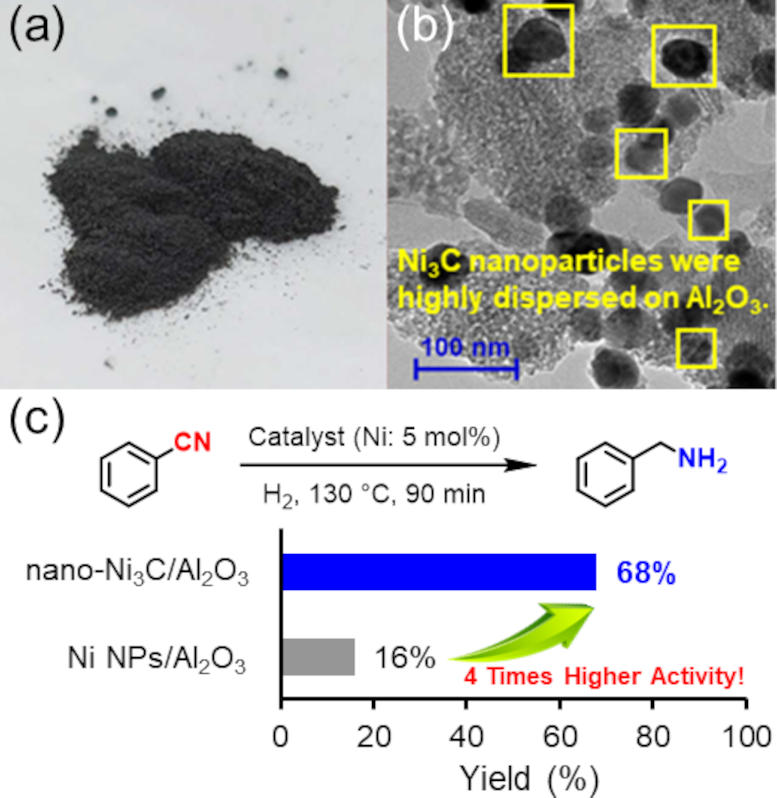
Unfortunately, many cheap metals require challenging experimental conditions, such as high pressures and temperatures, for the previously mentioned chemical transformation. Determining whether nickel carbide has the same limitations – and if not, evaluating the scope of the chemical transformations that are possible with this catalyst – was the goal of the research team’s study.
Research Findings and Catalyst Benefits
“In our work, we thoroughly study the reaction chemistry that underlies a novel nickel carbide nanoparticle heterogeneous catalyst for selective hydrogenation of nitriles into primary amines,” explains Sho Yamaguchi, lead author of the study. “The substrate scope is broad – many types of heteroaromatic and aliphatic nitriles can undergo this transformation.”
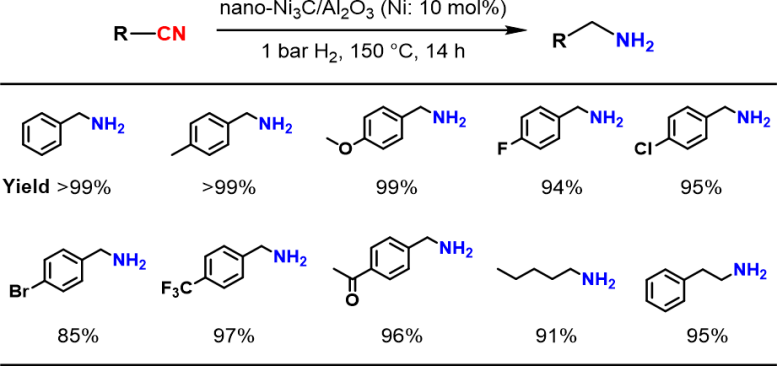
There are several advantages of the researchers’ catalyst. One, despite the mild required reaction conditions – 1-atmosphere pressure of hydrogen and a relatively low temperature of approximately 150°C – the catalyst still exhibited 4 times the activity of simple nickel nanoparticles. Two, the catalyst was reusable: at least 3-times. Three, the reaction yields were high: up to 99%.
“We’re excited because our research will help minimize the use of expensive metals for and simplifies the experimental setup of a common class of chemical syntheses,” says Tomoo Mizugaki, senior author. “Furthermore, our theoretical calculations provide insights that will help us optimize the catalyst for additional applications.”
This work is an important step forward in increasing the sustainability of a class of chemical reactions that are required for synthesizing pharmaceuticals and many other everyday products. Because the nickel catalyst is much cheaper than a noble metal, and the required experimental procedures are simple, feasible applications to further chemical transformations should be straightforward.
Reference: “Nickel Carbide Nanoparticle Catalyst for Selective Hydrogenation of Nitriles to Primary Amines” by Sho Yamaguchi, Daiki Kiyohira, Kohei Tada, Taiki Kawakami, Akira Miura, Takato Mitsudome and Tomoo Mizugaki, 5 January 2024, Chemistry – A European Journal.
DOI: 10.1002/chem.202303573
The study was funded by the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology.