Over the last decade, the photoelectrochemical (PEC) process for overall water-splitting (OWS) has seen comprehensive development, particularly in the areas of new catalysts, methods of characterization,, and reaction mechanisms. When comparing the hydrogen evolution reaction (HER) to the oxygen evolution reaction (OER), the latter is considered the more challenging aspect of OWS, primarily due to its sluggish kinetics.
To reduce the bias consumption of photoanodes, a series of OER alternative half-reactions have been developed, such as alcohols, urea, and ammonia oxidation reactions. The ultimate goal of photochemistry is to fabricate an efficient two-electrode unbiased PEC cell. However, most of the previous studies only focused on the properties of the working electrode in the three-electrode cell, while the polarization on the counter electrode was largely ignored. The synergistic mechanism between anodic oxidation and cathodic reduction half-reactions is still unclear.
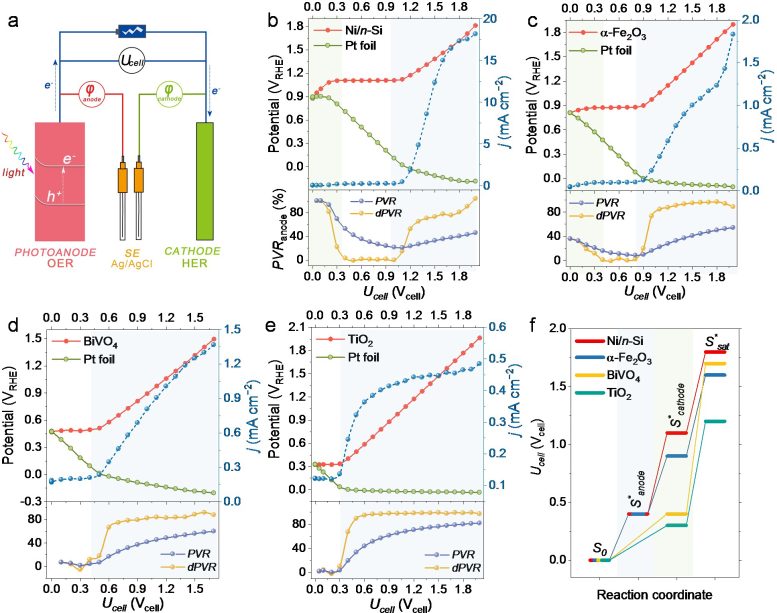
Recently, Professor Yuchao Zhang’s group proposed an experimental method to measure the bias distribution in a two-electrode PEC cell, systematically studying the bias distribution between representative photoanodes and Pt cathodes in PEC OWS cells. For the first time, they showed that the OER half-reaction is not always the rate-limiting factor of the OWS, and the bias consumption of electrodes depends on the photovoltage of the photoanode and the Fermi level of the cathode.
Further studies by using Ni/n-Si as the model photoanode showed that the bias distribution in the overall reaction can be effectively adjusted by tuning the electrolyte pH and coupled half-reactions. Accordingly, they proposed a descriptor to evaluate the compatibility between various half-reactions, which pointed out a general method for designing an efficient PEC overall reaction cell. Inspired by this, they fabricated an unbiased PEC cell consisting only of a Ni/n-Si photoanode and a Pt cathode with a photocurrent of 5.3 ± 0.2 mA cm−2.
Reference: “Bias distribution and regulation in photoelectrochemical overall water-splitting cells” by Kun Dang, Siqin Liu, Lei Wu, Daojian Tang, Jing Xue, Jiaming Wang, Hongwei Ji, Chuncheng Chen, Yuchao Zhang and Jincai Zhao, 6 February 2024, National Science Review.
DOI: 10.1093/nsr/nwae053